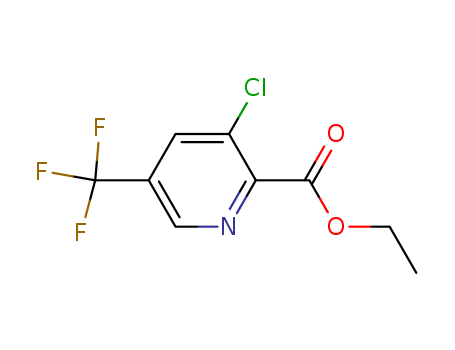
128073-16-5
- Product Name:Ethyl 3-chloro-5-trifluoromethylpyridine-2-carboxylate
- Molecular Formula:C9H7ClF3NO2
- Purity:99%
- Molecular Weight:253.608
Product Details
Purity:99%
Manufacturer supply top purity Ethyl 3-chloro-5-trifluoromethylpyridine-2-carboxylate 128073-16-5 with GMP standards
- Molecular Formula:C9H7ClF3NO2
- Molecular Weight:253.608
- Vapor Pressure:0.0047mmHg at 25°C
- Refractive Index:1.466
- Boiling Point:276.8 °C at 760 mmHg
- PKA:-3.19±0.10(Predicted)
- Flash Point:121.2 °C
- PSA:39.19000
- Density:1.389 g/cm3
- LogP:2.93050
2-PYRIDINECARBOXYLIC ACID, 3-CHLORO-5-(TRIFLUOROMETHYL)-, ETHYL ESTER(Cas 128073-16-5) Usage
|
General Description |
2-Pyridinecarboxylic acid, 3-chloro-5-(trifluoromethyl)-, ethyl ester is a chemical compound that is commonly used as a synthetic intermediate in the production of agricultural chemicals and pharmaceuticals. It is a chloro-substituted derivative of the aromatic compound pyridinecarboxylic acid, and the ethyl ester group enhances its solubility and stability. The presence of the trifluoromethyl group also provides unique chemical reactivity and physical properties, making it suitable for a wide range of chemical applications. 2-PYRIDINECARBOXYLIC ACID, 3-CHLORO-5-(TRIFLUOROMETHYL)-, ETHYL ESTER is important in the development of new materials and drugs, and its synthesis and properties continue to be the subject of research and development in the chemical industry. |
InChI:InChI=1/C9H7ClF3NO2/c1-2-16-8(15)7-6(10)3-5(4-14-7)9(11,12)13/h3-4H,2H2,1H3
128073-16-5 Relevant articles
3 - chloro -5 - trifluoromethyl pyridine compounds and intermediates method (by machine translation)
-
, (2017/07/20)
The invention discloses a 3 - chloro -5 ...
HETEROARYL SUBSTITUTED HETEROCYCLYL SULFONES
-
Page/Page column 87, (2015/11/09)
The invention relates to aryl substitute...
Herbicides
-
, (2008/06/13)
Compounds of formula (I) in which A is ═...
Pyrazole derivatives as herbicides
-
, (2008/06/13)
Compounds of the following formula I whe...
128073-16-5 Process route
-
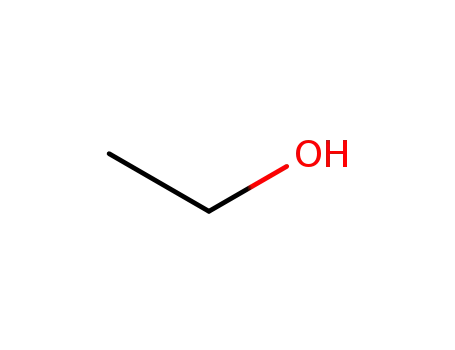
-
64-17-5
ethanol

-
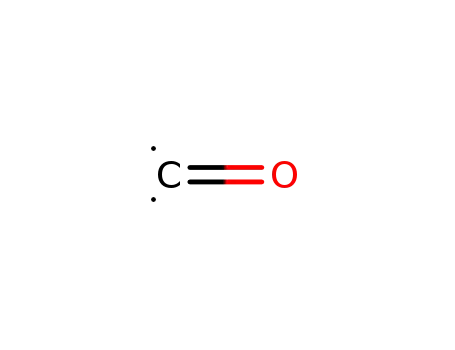
-
201230-82-2
carbon monoxide

-
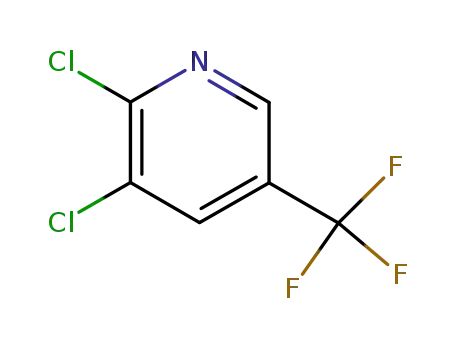
-
69045-84-7
2,3-dichloro-5-trifluoromethylpyridine

-
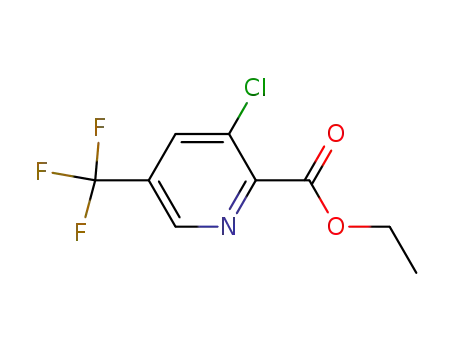
-
128073-16-5
3-chloro-5-trifluoromethylpyridine-2-carboxylic acid ethyl ester

-
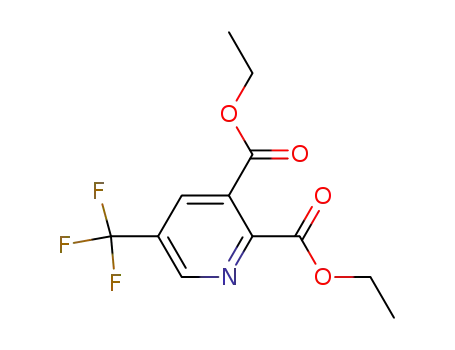
-
120083-60-5
diethyl 5-(trifluoromethyl)pyridine-2,3-dicarboxylate
| Conditions | Yield |
|---|---|
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium acetate; 1,4-di(diphenylphosphino)-butane;
at 120 ℃;
for 2h;
under 11400.8 Torr;
Autoclave;
|
32 %Chromat. 9 %Chromat. |
-

-
69045-84-7
2,3-dichloro-5-trifluoromethylpyridine

-

-
128073-16-5
3-chloro-5-trifluoromethylpyridine-2-carboxylic acid ethyl ester
| Conditions | Yield |
|---|---|
|
With
carbon monoxide; triethylamine;
In
ethanol;
|
85% |
|
With
triethylamine;
In
ethanol;
|
85% |
|
With
triethylamine;
In
ethanol; hexane;
|
|
|
Multi-step reaction with 3 steps
1.1: potassium fluoride / dimethyl sulfoxide / 115 - 120 °C / Inert atmosphere
2.1: tetrabutylammomium bromide / dimethyl sulfoxide; water / 45 - 50 °C
3.1: hydrogenchloride; pyridine / 19 h / 0 - 80 °C
3.2: 2 h / 25 - 30 °C
With
pyridine; hydrogenchloride; potassium fluoride; tetrabutylammomium bromide;
In
water; dimethyl sulfoxide;
3.1: |Pinner Imino Ether Synthesis;
|
128073-16-5 Upstream products
-
69045-84-7

2,3-dichloro-5-trifluoromethylpyridine
-
64-17-5

ethanol
-
201230-82-2
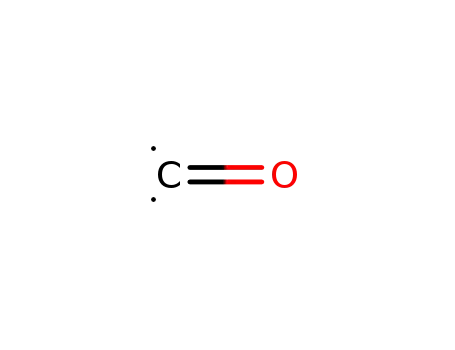
carbon monoxide
-
80194-70-3
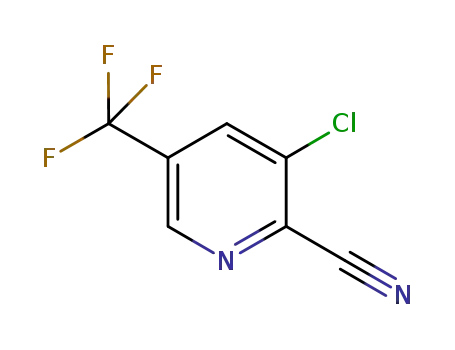
3-chloro-2-cyano-5-(trifluoromethyl)pyridine
128073-16-5 Downstream products
-
80194-68-9
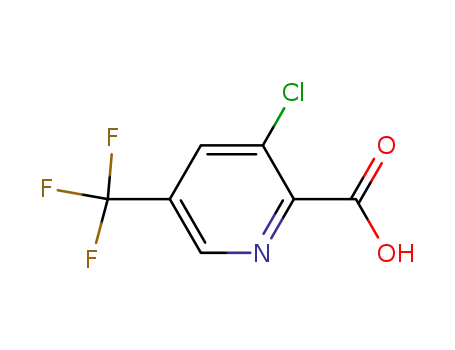
3-chloro-5-trifluoromethylpyridine-2-carboxylic acid
-
172527-65-0
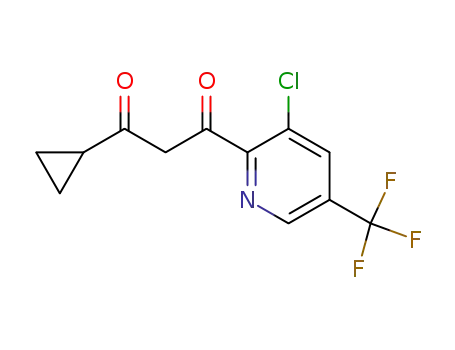
1-(3-chloro-5-trifluoromethylpyridin-2-yl)-3-cyclopropylpropan-13-dione
-
1245784-27-3
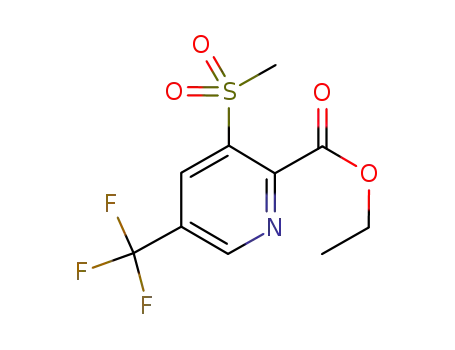
ethyl 3-methanesulfonyl-5-trifluoromethylpyridine-2-carboxylate
-
1033463-30-7
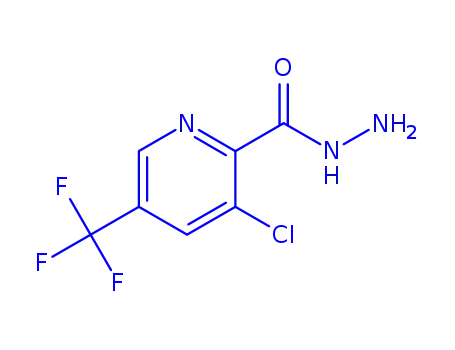
3-chloro-5-(trifluoromethyl)picolinohydrazide
Relevant Products
-
3-chloro-2-cyano-5-trifluoromethylpyridine
CAS:80194-70-3
-
2-Fluoro-3- (trifluoromethyl) benzoic acid
CAS:115029-22-6
-
1- (3-bromopyridin-2-yl) ethanone
CAS:111043-09-5