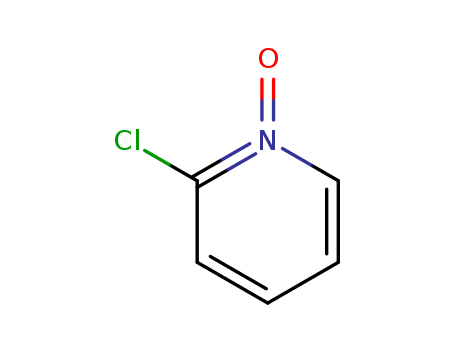
2402-95-1
- Product Name:2-chloropyridine-N-oxide
- Molecular Formula:C5H4ClNO
- Purity:99%
- Molecular Weight:129.546
Product Details
pd_meltingpoint:67-70 °C
Purity:99%
Manufacturer Supply Best Quality 2-chloropyridine-N-oxide 2402-95-1 with Efficient Transportation
- Molecular Formula:C5H4ClNO
- Molecular Weight:129.546
- Vapor Pressure:0.000733mmHg at 25°C
- Melting Point:67-70 °C
- Refractive Index:1,531-1,533
- Boiling Point:310 °C at 760 mmHg
- PKA:-0.76±0.10(Predicted)
- Flash Point:141.3 °C
- PSA:25.46000
- Density:1.27 g/cm3
- LogP:1.76850
2-Chloropyridine-N-oxide(Cas 2402-95-1) Usage
InChI:InChI=1/C5H3ClFNO/c6-5-4(7)2-1-3-8(5)9/h1-3H
Company Profile
Bailong Pharmaceutical officially began operations in 2017, located in the High-tech Zone of Jinan City. It is a high-tech enterprise specializing in the research and production of chemical raw materials and advanced pharmaceutical intermediates, and has currently passed the GB/T 19001-2016/ISO 9001 quality management system certification standards. The company is dedicated to serving major pharmaceutical companies, fine chemical production units, large pharmaceutical research and development centers, and other users of pharmaceutical chemicals both domestically and internationally. It has established cooperation with nearly all of the top ten pharmaceutical companies in the country and hundreds of small and medium-sized biotechnology companies, providing specialized services such as the supply of commercial chemicals, technical research and development, production technology outsourcing, and customized chemicals. In 2020, it was included in the list of "Top Ten Suppliers of the Year" for certain A-share listed companies, reaching a long-term cooperation intention to help clients improve production capacity and development efficiency, and push the project to market at full speed.

Development History
- 2020yearThe application for Shandong high-end pharmaceutical intermediates production base was successful, and it was officially put into operation in June 2022
- 2021yearIn the first quarter, the growth rate reached a new high, with a year-on-year increase of 210%, achieving leapfrog growth in the new fiscal year
- 2022yearIn December,the independent R&D laboratory building of Yinfeng Bio-City was put into operation to help product R&D and innovation.
2402-95-1 Relevant articles
Au(I)-Catalyzed Oxidative Functionalization of Yndiamides
Tong, Zixuan,Garry, Olivia L.,Smith, Philip J.,Jiang, Yubo,Mansfield, Steven J.,Anderson, Edward A.
supporting information, p. 4888 - 4892 (2021/06/28)
Yndiamides, underexplored cousins of yna...
From Pyridine- N-oxides to 2-Functionalized Pyridines through Pyridyl Phosphonium Salts: An Umpolung Strategy
Bugaenko, Dmitry I.,Yurovskaya, Marina A.,Karchava, Alexander V.
supporting information, p. 6099 - 6104 (2021/08/03)
The reactions of pyridine-N-oxides with ...
Recyclable anhydride catalyst for H2O2 oxidation:: N -oxidation of pyridine derivatives
Gajeles, Ghellyn,Kim, Se Mi,Lee, Kyung-Koo,Lee, Sang Hee,Yoo, Jong-Cheol
, p. 9165 - 9171 (2020/03/13)
The catalytic efficiency and recyclabili...
Method for catalyzing vitamin A isomerization with ruthenium catalyst
-
Paragraph 0050; 0053-0054, (2020/04/22)
The invention provides a method for cata...
2402-95-1 Process route
-
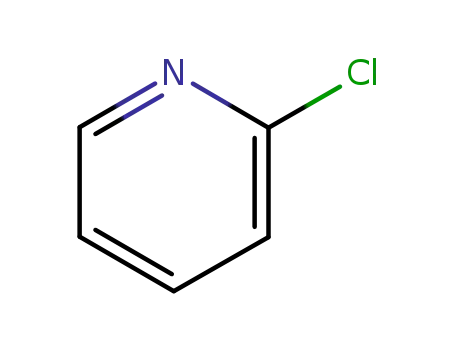
- 109-09-1
2-chloropyridine

-
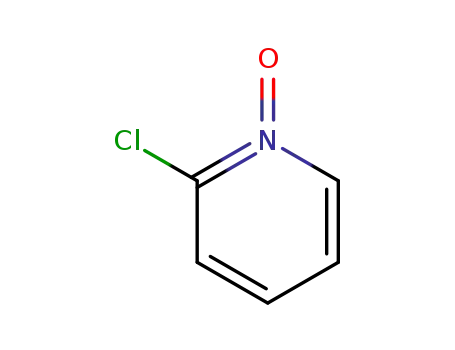
- 2402-95-1
2-chloropyridine-N-oxide
| Conditions | Yield |
|---|---|
|
With dihydrogen peroxide; In water; at 65 - 80 ℃; for 1h; Reagent/catalyst;
|
98.88% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane;
|
97% |
|
With 5% WO3/TiO2; dihydrogen peroxide; at 20 ℃; for 18h;
|
94.8% |
|
With dihydrogen peroxide; In water; at 90 ℃; for 7h; Reagent/catalyst;
|
93% |
|
With phthalic anhydride; urea-hydrogen peroxide; In acetonitrile; for 3h; Ambient temperature;
|
92% |
|
With dihydrogen peroxide; In trifluoroacetic acid; for 4h;
|
91% |
|
With titanium silicate; dihydrogen peroxide; In methanol; at 60 ℃; for 24h;
|
91% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20 ℃;
|
87% |
|
With trichloroisocyanuric acid; sodium acetate; acetic acid; In dichloromethane; water; acetonitrile; at 40 ℃; for 2h;
|
80% |
|
With dihydrogen peroxide; acetic acid; at 80 ℃; for 12h;
|
75% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20 ℃;
|
75% |
|
With sulfuric acid; dihydrogen peroxide; acetic acid; at 70 ℃; for 3h;
|
70% |
|
With HOF* CH3CN; In chloroform; at 0 - 20 ℃;
|
70% |
|
With dihydrogen peroxide; sodium hydrogencarbonate; trichloroacetonitrile; In tetrahydrofuran; water; at 0 - 25 ℃; for 12h;
|
70% |
|
With 3-chloro-benzenecarboperoxoic acid; In chloroform; at 25 ℃; for 10h;
|
68% |
|
With phthalic anhydride; urea hydrogen peroxide adduct; In ethyl acetate; at 100 ℃;
|
68% |
|
With 3-chloro-benzenecarboperoxoic acid; In chloroform; at 25 ℃; for 10h;
|
68% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20 ℃; for 4h;
|
67% |
|
With 3-chloro-benzenecarboperoxoic acid; In chloroform; at 65 - 70 ℃; for 24h;
|
60% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 24 ℃; for 12h; Inert atmosphere;
|
60% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 0 - 20 ℃;
|
53% |
|
With dihydrogen peroxide; acetic anhydride; In water; at 80 ℃; for 12h;
|
51% |
|
2-chloropyridine; In aq. phosphate buffer; at 30 ℃; for 8h; pH=7; Microbiological reaction; Green chemistry; Enzymatic reaction;
With D-glucose; at 30 ℃; chemoselective reaction; Microbiological reaction; Green chemistry;
|
43% |
|
With K8[α-BHW11O39]*13H2O; dihydrogen peroxide; In water; at 65 ℃; for 6h; chemoselective reaction; Green chemistry;
|
38% |
|
With 3-chloro-benzenecarboperoxoic acid; In chloroform; Heating / reflux;
|
37% |
|
With dihydrogen peroxide; Aliquat 336; In water; at 90 ℃; for 5.5h; Time; Reagent/catalyst; Reflux;
|
35% |
|
With (dimethyldioctadecylammonium)8 [HBW11O39]; dihydrogen peroxide; In tert-butyl alcohol; at 65 ℃; for 6h;
|
33% |
|
With △-Na8H[PW9O34]·19H2O; dihydrogen peroxide; In water; at 20 ℃; for 24h; Green chemistry;
|
26% |
|
With dihydrogen peroxide; In water; at 20 ℃; for 16h; Catalytic behavior;
|
24% |
|
With dihydrogen peroxide; Na12[WZn3(H2O)2(ZnW9O34)2]; at 75 ℃; for 7h;
|
10% |
|
With peracetic acid; acetic acid;
|
|
|
With dihydrogen peroxide; acetic acid;
|
|
|
With dihydrogen peroxide;
|
|
|
With MCPA; In dichloromethane; for 20h; Ambient temperature;
|
|
|
With magnesium monoperoxyphthalate hexahydrate; In acetic acid; at 85 ℃; for 2h; Yield given;
|
|
|
With dihydrogen peroxide; In acetic anhydride;
|
|
|
With dihydrogen peroxide; acetic acid; at 60 ℃; for 48h;
|
|
|
With dihydrogen peroxide; methyltrioxorhenium(VII); In dichloromethane; water; for 40h; Ambient temperature;
|
52 % Spectr. |
|
With 3-chloro-benzenecarboperoxoic acid;
|
|
|
With dihydrogen peroxide; sodium hydrogensulfite; In water; acetic acid;
|
|
|
With dihydrogen peroxide; In 1,4-dioxane; water; at 65 ℃; for 6h;
|
55 %Chromat. |
|
With dihydrogen peroxide; In water; at 20 ℃; for 24h;
|
43 %Chromat. |
|
With Candida antarctica lipase B; D-glucose; glucose oxidase from A. Niger; oxygen; In aq. phosphate buffer; ethyl acetate; at 20 ℃; for 1h; Green chemistry;
|
|
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane;
|
|
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20 ℃;
|
|
|
With dihydrogen peroxide; titanium(IV) oxide; at 20 - 50 ℃; for 10h; Temperature;
|
|
|
With dihydrogen peroxide; In water; at 65 - 80 ℃; for 2h; Molecular sieve; Green chemistry;
|
|
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20 ℃;
|
|
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 0 - 20 ℃; for 24h;
|
|
|
2-chloropyridine; With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 0 - 20 ℃; for 24h;
With potassium carbonate; In dichloromethane; water; at 20 ℃; for 0.5h;
|
-
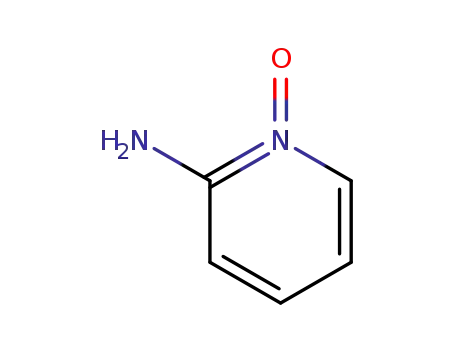
- 14150-95-9
2-aminopyridine N-oxide

-

- 2402-95-1
2-chloropyridine-N-oxide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: concentrated sulfuric acid; peroxomonosulfuric acid
With caro's acid; sulfuric acid;
|
2402-95-1 Upstream products
-
109-09-1

2-chloropyridine
-
2403-02-3
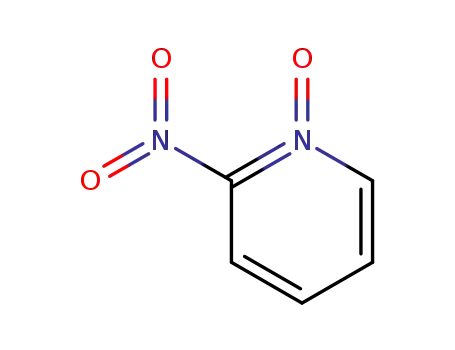
4-carboxy-2-nitropyridine N-oxide
-
75-36-5
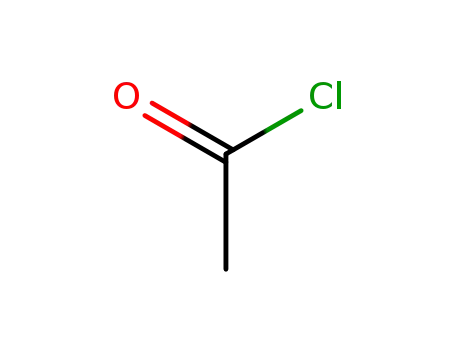
acetyl chloride
-
14150-95-9

2-aminopyridine N-oxide
2402-95-1 Downstream products
-
20773-98-2
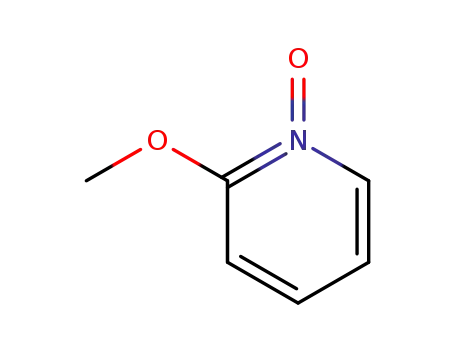
2-methoxypyridine N-oxide
-
3618-79-9
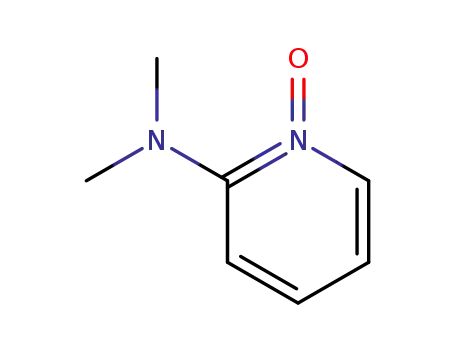
2-(dimethylamino)pyridine 1-oxide
-
79076-97-4
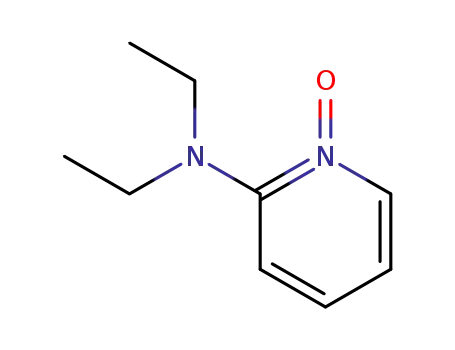
2-diethylaminopyridine N-oxide
-
54818-70-1
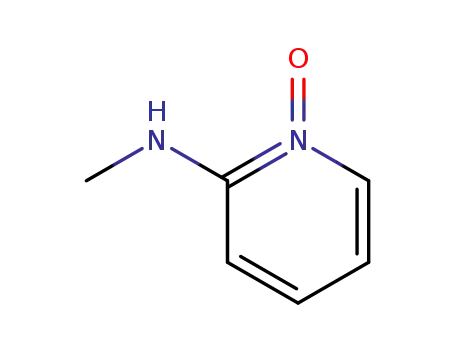
methyl-(1-oxy-pyridin-2-yl)-amine
Relevant Products
-
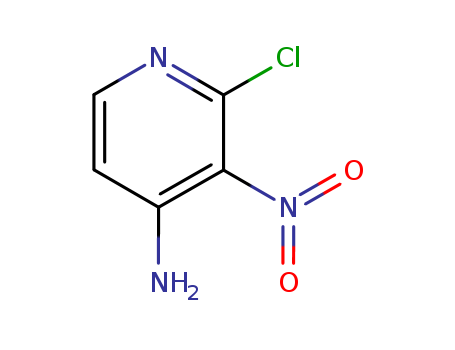
2-chloro-4-amino-3-nitropyridine
CAS:2789-25-5
-
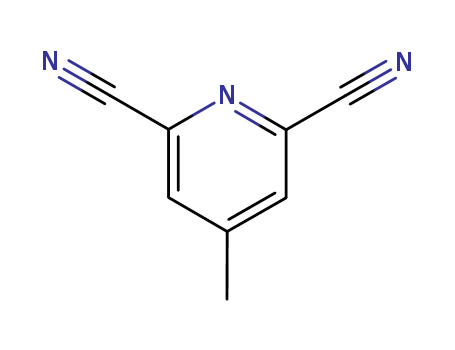
2,6-Dicyano-4-methylpyridine
CAS:21635-92-7
-
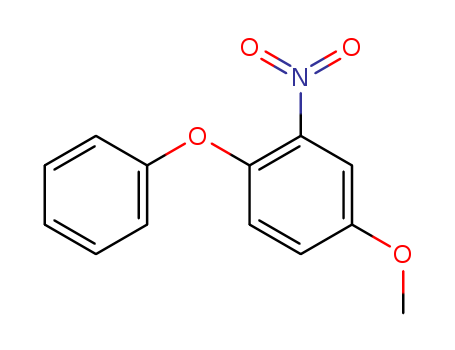
4-methoxy-2-nitro-1-phenoxybenzene (Impurity 5 of Eramod)
CAS:84594-95-6